About ZULRESSO
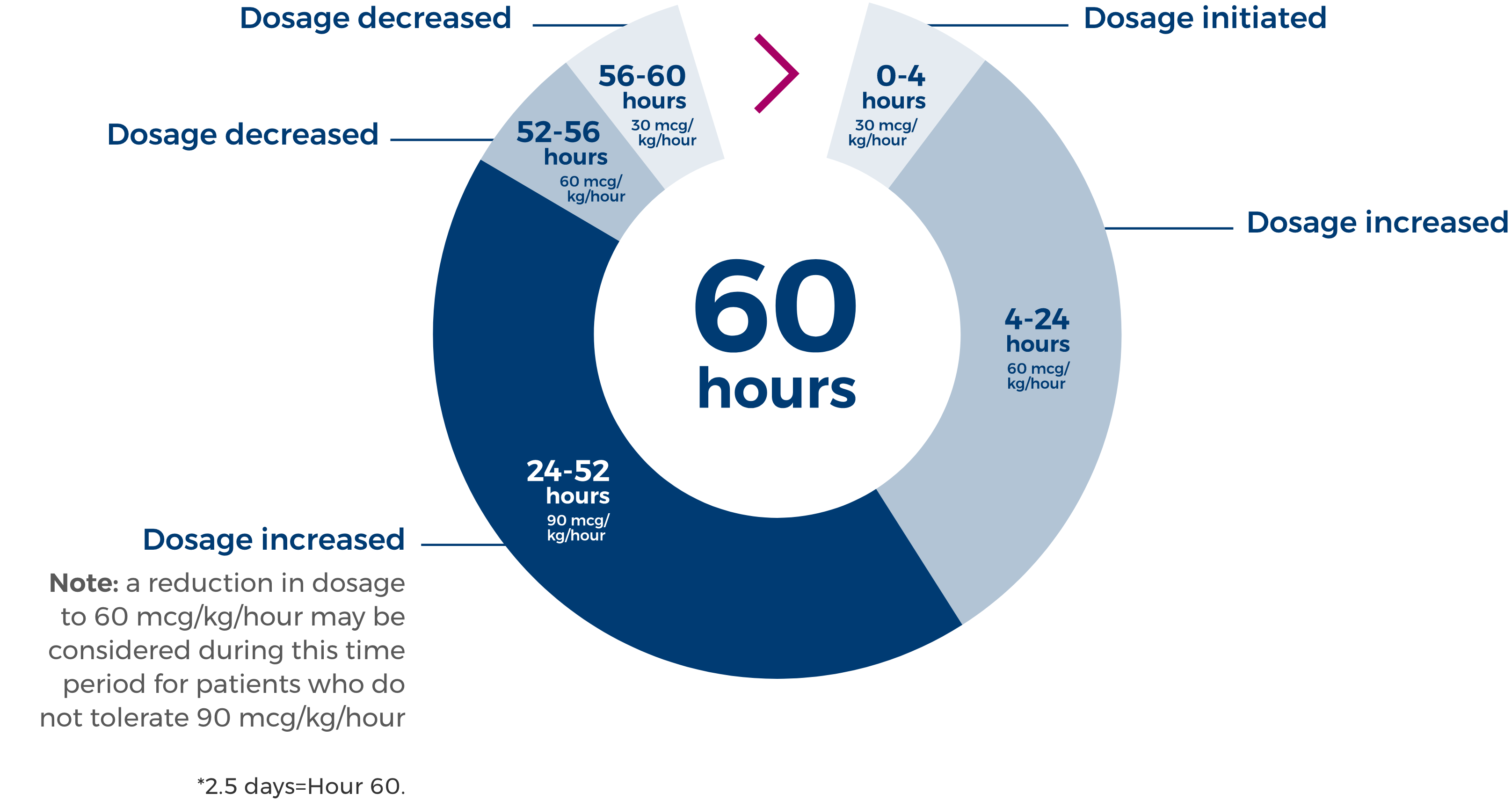
Dosing and administration with ZULRESSO
Recommended dosage is a titration to 90 mcg/kg/hour.
This link will take you to another Sage Therapeutics, Inc. website.
Sage Therapeutics, Inc. makes no representation as to the information contained on sites we do not own or control. Sage Therapeutics, Inc. does not recommend and does not endorse the content on any third-party websites. Your use of third-party websites is at your own risk and subject to the terms and conditions of use for such sites.

Individual results may vary.
Not actual patients.
About ZULRESSO
Recommended dosage is a titration to 90 mcg/kg/hour.
Avoid use of ZULRESSO in patients with end-stage renal disease (ESRD) with eGFR of <15 mL/minute/1.73 m2 because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
After dilution, the product can be stored in infusion bags under refrigerated conditions for up to 96 hours. However, given that the diluted product can be used for only 12 hours at room temperature, each 60-hour infusion will require the preparation of at least 5 infusion bags.

Visually inspect the vials of ZULRESSO for particulate matter and discoloration prior to administration. ZULRESSO is a clear, colorless solution. Do not use if the solution is discolored or particulate matter is present.
The 60-hour infusion will generally require the preparation of 5 infusion bags. Additional bags will be needed for patients weighing ≥90 kg.
For each infusion bag:
Prior to initiation and during therapy
Initiate ZULRESSO treatment early enough during the day to allow for recognition of excessive sedation.
A healthcare provider must be available onsite to continuously monitor the patient, and intervene as necessary, for the duration of the 60-hour (2.5 days) infusion.
Monitor patients for hypoxia using continuous pulse oximetry equipped with an alarm. Assess for excessive sedation every 2 hours during planned, non-sleep periods.
ZULRESSO must be diluted before administration.
Use a programmable peristaltic infusion pump to ensure accurate delivery of ZULRESSO.
Administer ZULRESSO via a dedicated line. Do not inject other medications into the infusion bag or mix with ZULRESSO.
Fully prime infusion administration sets with admixture before inserting into the pump and connecting to the venous catheter.
Use a PVC, non-DEHP, nonlatex infusion set. Do not use in-line filter infusion sets.
If excessive sedation occurs at any time during the infusion, stop the infusion until the symptoms resolve. The infusion may be resumed at the same or lower dose as clinically appropriate.
ZULRESSO is indicated for the treatment of postpartum depression (PPD) in patients 15 years and older.
WARNING: EXCESSIVE SEDATION AND SUDDEN LOSS OF CONSCIOUSNESS
Patients treated with ZULRESSO are at risk of excessive sedation or sudden loss of consciousness during administration.
Because of the risk of serious harm, patients must be monitored for excessive sedation and sudden loss of consciousness and have continuous pulse oximetry monitoring. Patients must be accompanied during interactions with their child(ren).
Because of these risks, ZULRESSO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZULRESSO REMS.
In clinical studies in adults, ZULRESSO caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of ZULRESSO-treated patients compared to 0% of placebo-treated patients). Some adult patients were also reported to have loss of consciousness or altered state of consciousness during the ZULRESSO infusion (4% of the ZULRESSO-treated patients compared with 0% of the placebo-treated patients). Time to full recovery from loss or altered state of consciousness, after dose interruption, ranged from 15 to 60 minutes in clinical studies in adults. A healthy 55-year-old man participating in a cardiac repolarization study experienced severe somnolence and <1 minute of apnea while receiving two times the maximum recommended dosage of ZULRESSO (180 mcg/kg/hour).
In an open-label clinical study in 20 patients ages 15 to 17 years, one patient experienced dizziness and loss of consciousness.
All patients with loss of or altered state of consciousness recovered with dose interruption. There was no clear association between loss or alteration of consciousness and pattern or timing of dose. Not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
During the infusion, monitor patients for sedative effects every 2 hours during planned, non-sleep periods. Immediately stop the infusion if there are signs or symptoms of excessive sedation. After symptoms resolve, the infusion may be resumed at the same or lower dose as clinically appropriate. Immediately stop the infusion if pulse oximetry reveals hypoxia. After hypoxia, the infusion should not be resumed.
Concomitant use of opioids, antidepressants, or other CNS depressants such as benzodiazepines or alcohol may increase the likelihood or severity of adverse reactions related to sedation. Patients must be accompanied during interactions with their child(ren) while receiving the infusion because of the potential for excessive sedation and sudden loss of consciousness.
Patients should be cautioned against engaging in potentially hazardous activities requiring mental alertness, such as driving, after infusion until any sedative effects of ZULRESSO have dissipated.
Notable requirements of the ZULRESSO REMS include:
Further information, including a list of certified healthcare facilities, is available at www.zulressorems.com or call 1-844-472-4379.
In pooled analyses of placebo-controlled trials of chronically administered antidepressant drugs (SSRIs and other antidepressant classes) that include approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with major depressive disorder (MDD).
ZULRESSO does not directly affect monoaminergic systems. Because of this and the comparatively low number of exposures to ZULRESSO, the risk of developing suicidal thoughts and behaviors with ZULRESSO is unknown. If depression becomes worse or patients experience emergent suicidal thoughts and behaviors, consider changing the therapeutic regimen, including discontinuing ZULRESSO.
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.
Adverse reactions reported in an open-label study in patients 15 to 17 years were generally similar to those observed in clinical studies of ZULRESSO in adults with PPD.
ZULRESSO contains brexanolone, a Schedule IV controlled substance under the Controlled Substances Act.
Please also see Full Prescribing Information including Boxed Warning and Medication Guide for ZULRESSO.